is ethyl alcohol polar Alcohols and ethers
Hey there! Today, we’re going to dive into the fascinating world of alcohols and ethers. These compounds play a significant role in various industries and have some intriguing properties. Let’s explore!
Alcohols and Ethers: A Closer Look
Alcohols are organic compounds that contain the hydroxyl (-OH) functional group. They are derived from hydrocarbons by substituting one or more hydrogen atoms with hydroxyl groups. One example of an alcohol is ethanol (C2H5OH), which you might be familiar with as it is commonly found in alcoholic beverages.
 Ethers, on the other hand, are organic compounds characterized by an oxygen atom bonded to two alkyl or aryl groups. They have a unique structure that sets them apart from alcohols. One notable example is diethyl ether, which was commonly used as an anesthetic in the past.
Ethers, on the other hand, are organic compounds characterized by an oxygen atom bonded to two alkyl or aryl groups. They have a unique structure that sets them apart from alcohols. One notable example is diethyl ether, which was commonly used as an anesthetic in the past.
Exploring Ethanol: Polar or Nonpolar?
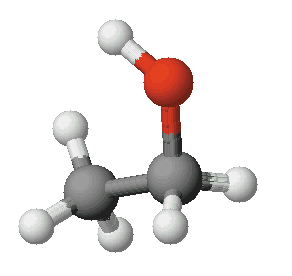
Let’s zoom in on ethanol, a widely known alcohol. Have you ever wondered if it is polar or nonpolar? Polar molecules have an uneven distribution of charge and contain polar bonds, while nonpolar molecules have an equal sharing of electrons and contain nonpolar bonds.
 In the case of ethanol, it is considered a polar molecule. The oxygen atom in the hydroxyl group attracts electrons more strongly than the carbon and hydrogen atoms. This creates a partial negative charge on the oxygen atom and partial positive charges on the carbon and hydrogen atoms. As a result, ethanol displays polarity.
In the case of ethanol, it is considered a polar molecule. The oxygen atom in the hydroxyl group attracts electrons more strongly than the carbon and hydrogen atoms. This creates a partial negative charge on the oxygen atom and partial positive charges on the carbon and hydrogen atoms. As a result, ethanol displays polarity.
The polarity of ethanol influences its properties and behavior. For instance, ethanol is miscible with water, meaning it can mix evenly with water in all proportions. This property makes it a vital ingredient in many alcoholic beverages, pharmaceuticals, and personal care products.
Additionally, ethanol’s polarity allows it to dissolve various polar substances like salts, sugars, and organic acids. This solubility property makes ethanol an excellent solvent for certain applications, such as extracting plant compounds or sanitizing surfaces.
On the other hand, ethanol’s polarity also contributes to its high boiling point and viscosity compared to nonpolar compounds with similar molecular weights. These characteristics are critical in applications like fuel, where ethanol is blended with gasoline.
In conclusion, alcohols and ethers are versatile compounds that play pivotal roles in various industries. Ethanol, as an alcohol, exhibits polarity due to the presence of the hydroxyl group. This property allows ethanol to mix with water and dissolve polar substances. Its unique characteristics make it a valuable component in numerous products we encounter in our daily lives.
So, the next time you enjoy a refreshing beverage or use a personal care product, spare a thought for the interesting science behind alcohols and ethers! Cheers!
If you are searching about Lab: Solubility of a Salt in a Polar and a Non-polar Solvent you’ve visit to the right page. We have 5 Pics about Lab: Solubility of a Salt in a Polar and a Non-polar Solvent like Lab: Solubility of a Salt in a Polar and a Non-polar Solvent, Is Ethanol (C2H5OH) Polar or Nonpolar? - Techiescientist and also Is Ethanol (C2H5OH) Polar or Nonpolar? - Techiescientist. Read more:
Lab: Solubility Of A Salt In A Polar And A Non-polar Solvent
 kaffee.50webs.comalcohol ethyl gif
kaffee.50webs.comalcohol ethyl gif
Is Ethanol (C2H5OH) Polar Or Nonpolar? - Techiescientist
 techiescientist.comethanol ethyl chemical etanol polar nonpolar funziona alcol kimia techiescientist distillation ethylalcohol praktikum organik polarity
techiescientist.comethanol ethyl chemical etanol polar nonpolar funziona alcol kimia techiescientist distillation ethylalcohol praktikum organik polarity
Vector Organic Polar Aliphatic Molecule Or Solvent Of Ethanol C2H6O Or
 imagemart.aflo.comAlcohols And Ethers
imagemart.aflo.comAlcohols And Ethers
 www.4college.co.ukwater alcohols alcohol polar group hydrogen ethers molecules alkyl atoms replaced been
www.4college.co.ukwater alcohols alcohol polar group hydrogen ethers molecules alkyl atoms replaced been
Is Ethanol (C2H5OH) Polar Or Nonpolar? - Techiescientist
 techiescientist.comethanol nonpolar techiescientist ethyl
techiescientist.comethanol nonpolar techiescientist ethyl
Alcohols and ethers. Alcohol ethyl gif. Is ethanol (c2h5oh) polar or nonpolar?